- About
- Organization
- Organization Overview
- Dean’s Office
- Department of Bioengineering and Therapeutic Sciences
- Department of Clinical Pharmacy
- Department of Pharmaceutical Chemistry
- Quantitative Biosciences Institute
- Org Chart
- Research
- Education
- Patient Care
- People
- News
- Events
Data-driven drug dosing speeds tuberculosis treatment to four months
UCSF research leads to breakthrough in treating the global pandemic
By Levi Gadye / Mon Aug 30, 2021
It’s a disease of the lungs. It spreads through the air. It can kill millions in a year. And it’s the cause of an ongoing pandemic.
The disease isn’t COVID-19. It is tuberculosis (TB). Each year, nearly ten million people are diagnosed with TB and over a million die. Prior to COVID, TB was the deadliest infectious disease on the planet. Yet the gold standard for treatment is a prolonged, daily regimen of multiple antibiotics, taken for a minimum of six months, that hasn’t changed in decades—a regimen that places a huge burden on patients already struggling to make ends meet.
A clinical trial of new treatment regimens, led in part by researchers at UCSF, recently demonstrated that a more potent combination of antibiotics could shorten the duration of treatment for TB8, giving patients back two months of their lives and improving their chances of taking every dose. The results from the trial were published on May 6 in the New England Journal of Medicine.
“The existing treatment for TB is six months long, it’s hard, and there are a lot of issues with it,” said UCSF School of Pharmacy faculty member Rada Savic, PhD9, whose work on TB drug pharmacology served as the basis for the trial. “The entire drug development process has focused for 40 years to try to shorten it, many trials have failed, and this is the first success.”
Findings from the trial were so robust that the World Health Organization (WHO) endorsed the regimen this summer10, enabling health care providers and TB programs around the world to use it.
“This landmark trial was the largest randomized, registration-quality trial of new regimens for pulmonary TB to date,” said Payam Nahid, MD, MPH11, who served as the clinical trial protocol co-chair and serves as director of the UCSF Center for Tuberculosis12. “Rada’s thorough data analyses gave the team insight into how to optimize the dosages and duration of the regimen, and it worked. It’s a model for others to follow in infectious disease.”
Advances in TB treatment stalled in the 1980s
TB is one of the oldest infectious diseases known to afflict humans, with tests of human remains from 9,000 years ago turning up TB positive13.
But well into the 20th century, the medical field could do little for TB patients. In some countries, these patients were encouraged or even forced to quarantine in sanatoriums—facilities isolated from the population—that did nothing to treat the disease.

MMFE at Wikimedia Commons (cc-by-sa)
A sanatorium in Vianden, Luxembourg. Prior to the advent of antibiotic treatments, sanatoriums were used to quarantine patients with TB.
Vaccines and antibiotics against TB first emerged around the turn of the century but struggled to gain widespread use. A critical advance was made in the late 1940s when the British Medical Research Council methodically tested two antibiotics in patients with TB without knowing which patients were receiving which drugs—the first-ever randomized clinical trial.
The trial showed that treating TB with two drugs at once was much more effective than with either drug alone. But the combination caused debilitating side effects and required a commitment of two years of daily pills. Miss a few doses, and the infection would come roaring back.
Over time, more-potent antibiotics were substituted into the drug cocktail, reducing the duration of treatment from 24 to 12 to 9 to 8 to 6 months, according to Nahid. But that progress stalled 40 years ago, saddling millions of patients with half a year on the drugs.
“We’ve heard from TB survivors how a reduction in duration would be a huge benefit to them, to get back to their lives, to get back to being comfortable around their family, their children, their friends,” said Nahid.
Shortening the duration of the TB drug regimen also promised to free up some of the scarce resources used in the fight against the disease, which today is most prevalent in countries where health care is not always readily available. But clinical trials even in the late 2000s continued to come up short.
Following the data to the most effective dose
The successful clinical trial, known as Study 31/A534914, benefited from the lessons learned from recent, failed trials, combined with rigorous pharmacological modeling and data integration from the Savic Lab.
Savic, a faculty member in the Department of Bioengineering and Therapeutic Sciences15, a joint department of the Schools of Pharmacy and Medicine, is an expert in pharmacokinetics, or the study of how drugs move through the body. When it came to the six-month, four-drug regimen used to treat TB, experience had taught her to be skeptical that the drugs were being used to their greatest potential.

Rada Savic, PhD, scrutinized data from prior, failed clinical trials of drug regimens for TB and used computational models to design a faster treatment for the disease.
The six-month regimen had been designed in the 1970s around the lowest effective dose of its cornerstone antibiotic, rifampin, giving patients just enough medication to beat the disease. But TB is a disease in which the offending bacteria multiply out of control even in the presence of antibiotics, and many patients remain sick for years despite treatment.
Previous trials had attempted to replace rifampin with a more potent drug, rifapentine. Some of these trials with rifapentine made the dosing more intermittent—weekly, instead of daily—or upped the dosages in the final few months of the regimen. But none were as effective as the existing regimen with rifampin.
Savic wondered whether rifapentine could actually be given at higher doses, overwhelming the infection without hurting patients—a safe and more effective dose. Starting in 2012, Savic and her team reanalyzed the results from these failed clinical trials of rifapentine and made a pivotal discovery.
“When we rearranged the data, combining patients according to their raw doses of rifapentine, we started seeing beautiful dose responses to the drug,” said Savic. “When we went further and looked into pharmacokinetics and plasma levels [in the bloodstream], we again saw that patients with the highest drug exposure have the best response and are most likely to recover.”
Drugs are commonly dosed relative to the body weight of a patient—the smaller the patient, the thinking goes, the lower the dose. Yet in the field of pharmacokinetics, it’s well known that this logic does not always hold up against the complexity of the human body, as borne out by Savic’s work. The original analyses had missed this because they were analyzed according to patients’ relative dosages of the drug—not their absolute dosages.
“I was not surprised by my findings,” said Savic. “As a pharmacologist and pharmacist, I know there are better ways for finding the right dose, and our analysis was a textbook example of using the right approach to solve a dosing problem.”
In addition to testing an optimized dose of rifapentine, the team also sought to test a substitution for another drug in the cocktail, replacing ethambutol with the stronger moxifloxacin to account for patients with more persistent cases of TB infection lodged deep in the lungs. Like the rifapentine substitution, the moxifloxacin substitution had a body of evidence, from animal models and computer simulations, to back it.
Savic next needed to prove that these proposed changes in TB treatment could actually provide an effective and expedient cure in the real world.
Getting the largest TB trial off the ground
In 2013, Savic joined forces with Nahid, who directs the UCSF Center for Tuberculosis17 and has led a wide variety of research and policy efforts to combat TB both for UCSF and the WHO, including clinical trials of new drug regimens.
Nahid had conducted early phases of clinical trials for TB over the years, but the strength of Savic’s work lent new energy to get a large, phase 3 clinical trial for a new TB regimen off the ground. Phase 3 clinical trials represent the final bar that a treatment must clear in order to be approved. They involve hundreds to thousands of patients so there must be high confidence in the safety and effectiveness of a new therapy—a lot is on the line.

Payam Nahid, MD, MPH, director of the UCSF Center for Tuberculosis, co-led the successful clinical trial of new drug regimens for tuberculosis.
“The sophistication with pharmacometric, computational approaches, brought in by Rada’s team in collaboration with others, allowed for a rich dialogue in the design of the trial and the selection of the regimens, and made it easier to get our international colleagues on board,” said Nahid.
As investigators in the CDC-funded TB Trials Consortium18 (TBTC) and the NIH-funded AIDS Clinical Trials Group19 (ACTG), Nahid and Savic were able to engage with both groups' connections to TB experts and clinics around the world. The two worked collaboratively with a diverse international protocol team, convened by the TBTC and ACTG, to design and execute what they hoped would be a watershed trial.
“I’ve witnessed a lot of excellent pharmacokinetic work that doesn’t move the needle for patients,” said Savic. “My weapon was to keep showing the data to keep making the point, to build trust with the clinicians, to communicate our science in a way that people will accept and recognize the value.”
In designing Study 31/A5349, the growing village of experts considered that any eventual new therapy would be destined for a diverse population of millions of people with TB.
“We sought to put in features to make the trial as close to the real world as possible, while retaining the essential components that are needed for FDA approval,” said Nahid. “We allowed enrollment down to adolescence, 12 years and older, as well as patients living with advanced HIV, two populations that the most recent trials had overlooked.”
In January 2016, the trial began to enroll participants with TB and the first patients began their months of treatment.
From computer model to WHO recommendation
Ultimately 2,516 patients were enrolled across 34 test sites on four continents and were randomly assigned one of three regimens:
- A control, six-month regimen of rifampin, isoniazid, pyrazinamide, and ethambutol.
- A four-month regimen of rifapentine, isoniazid, pyrazinamide, and ethambutol.
- A four-month regimen of rifapentine, isoniazid, pyrazinamide, and moxifloxacin.
The trial followed each patient for 18 months to track whether they stayed TB-free for at least a year after the end of treatment.
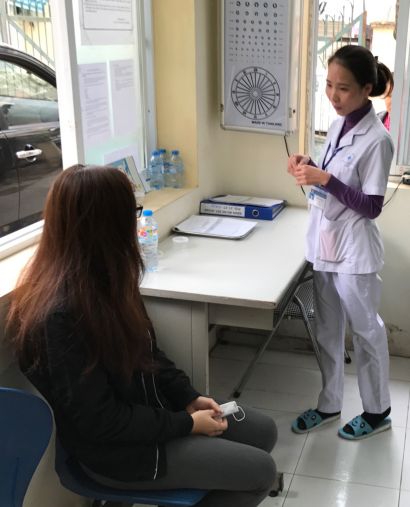
A Study 31/A5349 clinical trial participant, left, attends a follow-up appointment at a Vietnam National TB Programme and UCSF-partnered district health center, Hanoi, Vietnam.
The results bore out Savic’s predictions: the four-month regimen with a high dose of rifapentine along with moxifloxacin was shown to be just as effective and safe as the existing six-month regimen.
The results of the trial were so convincing that the World Health Organization convened a panel to review the data. In a rapid communication released in June, the WHO noted:
The 4-month regimen, which is shorter, effective and all-oral, would be a preference for many patients and also national TB programmes, allowing faster cure and easing the burden on both patients and the healthcare system... Shortened treatment has the potential to improve adherence and reduce patient and health system costs.
It will take years for the new regimen to become routine in clinics across the globe. TB has long been a disease of poverty, one that divides society by class and offers little to no financial incentive for industry to invest in research and development. But with the nod from the WHO, change is finally here for millions with TB.
For Savic and Nahid, the work continues. They are now exploring how to further reduce the duration to two-month regimens.
“We want to take the same regimen, with the same drugs, and give it only for two months in easy-to-treat patients who can be identified with simple field tests,” said Savic. “We have the evidence this will work, and we are working on a new round of clinical trials, rallying our collaborators again.”
Nahid is optimistic that the professional connections forged during Study 31/A5349 and the data-driven approach to drug dosing and regimen optimization will inspire more bold clinical trials for diseases that, like TB, continue to plague humanity.
“It’s been a shared common goal of so many of us to finally improve our therapies for TB,” said Nahid. “It took over a decade, but we said, ‘Let’s all come together and roll up our sleeves,’ even with limited funding, and we couldn’t be prouder of the result.”
More
- UCSF Center for Tuberculosis12
- Four-Month Rifapentine Regimens with or without Moxifloxacin for Tuberculosis (The New England Journal of Medicine)8
- Treatment of drug-susceptible tuberculosis: rapid communication (World Health Organization)10
- UCSF Co-led Study Demonstrates Safety and Efficacy of 4-Month Tuberculosis Treatment Regimen20
- Data for a difference21
Tags
Topics:
Category:
Research26, Patient Care27
Sites:
School of Pharmacy, Department of Bioengineering and Therapeutic Sciences, PharmD Degree Program
About the School: The UCSF School of Pharmacy aims to solve the most pressing health care problems and strives to ensure that each patient receives the safest, most effective treatments. Our discoveries seed the development of novel therapies, and our researchers consistently lead the nation in NIH funding. The School’s doctor of pharmacy (PharmD) degree program, with its unique emphasis on scientific thinking, prepares students to be critical thinkers and leaders in their field.




